PRODUCT IDENTIFICATION
25973-55-1
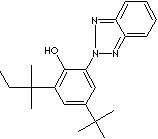
247-384-8
351.49
Oral rat LD50: 5000 mg/kg
SMILES
CLASSIFICATION
Benzotriazole, UV Absorber, Light stabilizer
PHYSICAL AND CHEMICAL PROPERTIES
Slightly yellowish powder
80 - 83 C
1.17
Insoluble (0.015 mg/l at 25 C)
REFRACTIVE INDEX
229 C
GENERAL DESCRIPTION & EXTERNAL LINKS
UV Absorber 328 has phenolic benzotriazole moiety and two substrates (tert-amylphenol) present on the phenolic group at 3,5 position. Phenolic Benzotriazole structure imparts important role in polymer and coating industry as UV absorbers, Light stabilizers. UV absorbers reduce or prevent the absorption of UV light by chromophores which in an excited state can form radicals that may have damaging effects on materials or alter their properties. UV Absorber 328 provides effective light stabilization and prevents yellowing and degradation of polymers such as polyurethanes, polyvinylchloride, unsaturated polyester and styrenics.
http://botkinchemie.com/
Ultraviolet
(UV) absorbers act largely by shielding the interior of the part
from damaging UV radiation. Before the advent of hindered amines,
light stabilization was accomplished with systems based on UV absorbers
in combination with other additives. Today UV absorbers are often
used in combination with HALS and sometimes other additives for
the stabilization of polypropylene. They are also used in plastic
packaging applications to provide content protection. All of the
compounds of this class share several general characteristics:
1. Strong UV absorption, especially in the 290 nm to 350 nm range which is very damaging to organic materials, and
2. Excited states formed upon UV absorption relax to the ground state extremely rapidly (picosecond time frame) and efficiently via radiation-less processes, which imparts high efficiency and excellent photostability.
Chemical classes of UV absorbers suitable for use in polyolefins include 2-hydroxybenzophenones (e.g.UVA-1), 2-(2-hydroxyphenyl)-2H-benzotriazoles (e.g. UVA-2, UVA-3), tris-aryl-o-hydroxyphenyl-striazines (e.g. UVA-4), and cyanoacrylate derivatives (e.g. UVA-5, UVA-6). Chemical structures of representative UV absorbers are given in the Appendix. For food contact applications, products that have been designated as food contact compliant in polypropylene by the US FDA include UVA-1, UVA-3, UVA-4, and UVA-6.........
APPEARANCE
MELTING POINT
80 C min
VOLATILES
0.5% max
95.0% (460nm), 97.0% (500nm)
1,2,3-Benzotriazole: Benzene ring-fused azole compounds; white to off-white crystalline powder; insoluble in water, soluble in ethanol, melting at 98.5 C. Azole is a five-membered heterocycle compound containing unsaturated bonds and Nitrogen atoms. Triazole has three nitrogens. 1,2,3-Benzotriazole (1H-benzotriazole) has -N=N- bond whereas 2H-Benzotriazole has two -C=N- bonds. Benzotriazole is a parent material to produce UV-absorbers. Benzotriazole and its derivatives are versatile intermediates involved in the production of:
- Corrosion Inhibitors
- Anti-fading agent for metals
- Antiseptic and Anticoagulant agent
- Anti-fog for photograph
- UV-absorbers
- Anti-freeze Agent
- Photoconductor
- Copying systems
- Pharmaceuticals, pesticide products and other specialty chemicals